Device-related complications in subcutaneous versus transvenous ICD: a secondary analysis of the PRAETORIAN trial
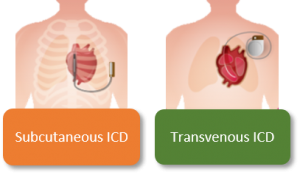
Some patients are at risk for inefficient fast heartrates that can – if not treated – result in death. Implantable cardioverter defibrillators (ICD) are a successful treatment. However, an ICD might also cause complications. The ICD exists of two components: a can (the device) and a lead. Currently two types of ICDs are used: the subcutaneous ICD (S-ICD) and transvenous ICD (TV-ICD), see figure. The latter uses a lead that is implanted in the heart (connecting via the blood vessels the lead with the device, which is usually positioned in the left upper chest region). The S-ICD, which uses a lead that is not in the blood vessels, is especially developed to overcome complications of the lead such as fractures or infections.
With the S-ICD, the lead is positioned on the outside of the thorax and the device is positioned below the left axilla. Therefore, the type of complications seen during and after implanting a TV-ICD differ from those seen during and after implanting an S-ICD. Also, the severity of complications can differ as some complications result in a longer hospital admission whereas others lead to a reoperation. In this study we described the different types of complications and the difference in severity of complications between the S-ICD and TV-ICD.
This study showed that bleedings were the most common complication in patients with an S-ICD. In patients with a TV-ICD, lead dysfunction and infection were the most common complications. In almost 2/3rd of the complications a new hospitalization was required. Patients with an irregular heartrate in the S-ICD group had a higher risk of developing a complication. Patients with a higher BMI in the S-ICD group had a higher risk of experiencing a complication that required a reoperation. Overall complications of the lead and severe infections were more common in patients with a TV-ICD. The complications were also more severe in patients with a TV-ICD as they required more frequently a reoperation. This data is important to optimally inform both patient and physician before making the choice between both devices, when both are suitable.
Translated by Shari Pepplinkhuizen, Amsterdam UMC, Amsterdam, The Netherlands.
Reinoud E. Knops, Shari Pepplinkhuizen, Peter Paul H.M. Delnoy, Lucas V.A. Boersma, Juergen Kuschyk, Mikhael F. El-Chami, Hendrik Bonnemeier, Elijah R. Behr, Tom F. Brouwer, Stefan Kaab, Suneet Mittal, Anne-Floor B.E. Quast, Willeke van der Stuijt, Lonneke Smeding, Jolien A. de Veld, Jan G.P. Tijssen, Nick R. Bijsterveld, Sergio Richter, Marc A. Brouwer, Joris R. de Groot, Kirsten M. Kooiman, Pier D. Lambiase, Petr Neuzil, Kevin Vernooy, Marco Alings, Timothy R. Betts, Frank A.L.E. Bracke, Martin C. Burke, Jonas S.S.G. de Jong, David J. Wright, Ward P.J. Jansen, Zachary I. Whinnett, Peter Nordbeck, Michael Knaut, Berit T. Philbert, Jurren M. van Opstal, Alexandru B. Chicos, Cornelis P. Allaart, Alida E. Borger van der Burg, Jose M. Dizon, Marc A. Miller, Dmitry Nemirovsky, Ralf Surber, Gaurav A. Upadhyay, Raul Weiss, Anouk de Weger, Arthur A.M. Wilde, and Louise R.A. Olde Nordkamp, and On behalf of the PRAETORIAN Investigators. Device-related complications in subcutaneous versus transvenous ICD: a secondary analysis of the PRAETORIAN trial. European Heart Journal (2022) 43(47), 4872-4883.
